The agency said that the delay was caused by non-compliance with essential quality assurance procedures and not an intentional blockage.
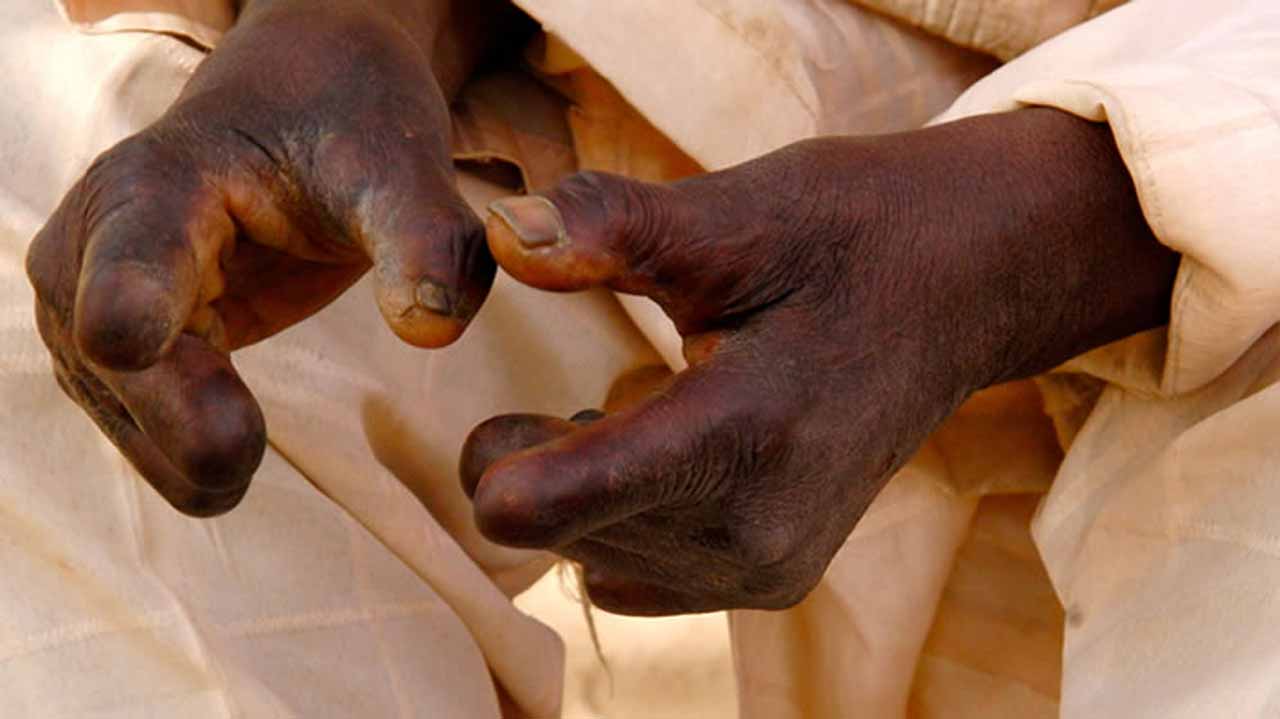
In a statement, yesterday, NAFDAC’s Director-General, Prof. Mojisola Adeyeye, said that the World Health Organisation (WHO) had requested a waiver for certain mandatory testing policies to allow the importation of Rifampicin, an anti-leprosy drug.
She said that the manufacturer failed to secure a Certificate of Pharmaceutical Product (CoPP) from the Indian Regulatory Authority, a key requirement under WHO guidelines.
The agency, therefore, confirmed that after receiving a laboratory evaluation report from an approved Clean Report of Inspection and Analysis (CRIA) laboratory in India, it approved the release of the drugs.
It, therefore, reaffirmed its commitment to public health, stating that it is working to reduce dependence on imported medicines by strengthening regulatory systems and supporting local pharmaceutical manufacturers, adding: “NAFDAC will continue to ensure that only quality, safe, and efficacious medicines are available for distribution, sale, and use within Nigeria.”






