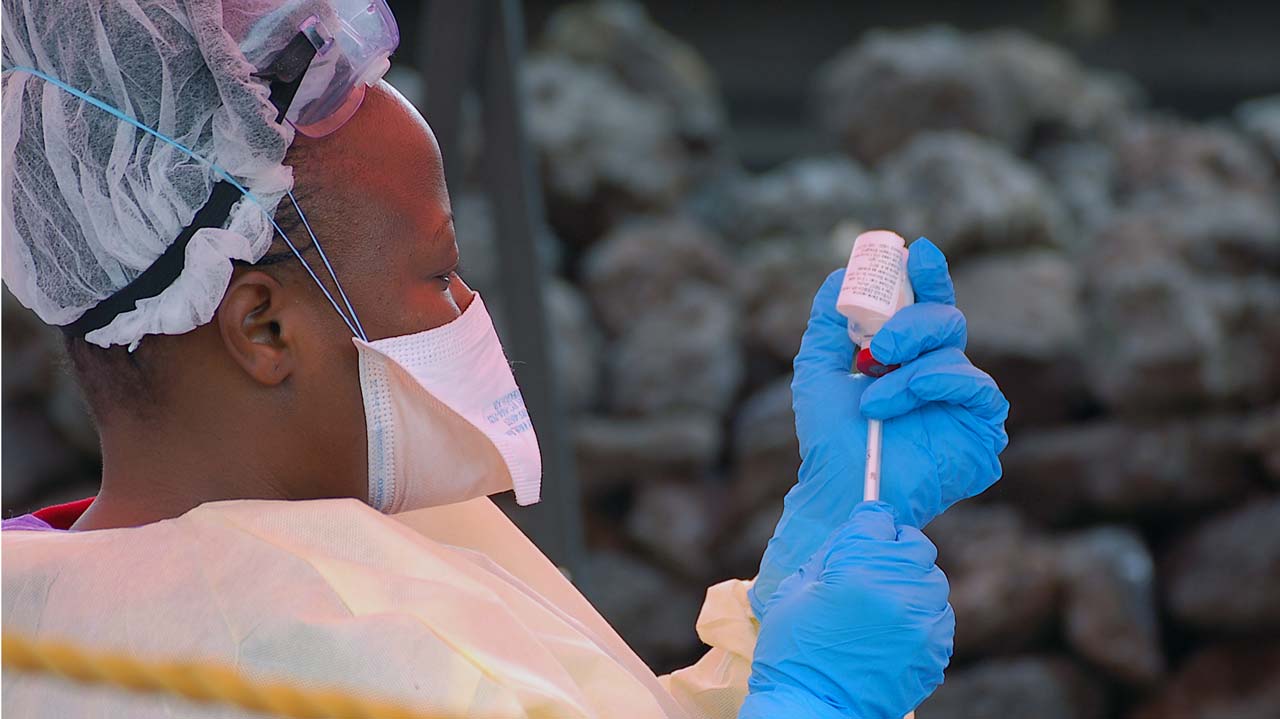
The Democratic Republic of Congo will introduce a second Ebola vaccine next month, the World Health Organization said Monday, as a top medical charity accused the UN agency of rationing doses.
DRC’s latest Ebola epidemic, which began in August 2018, has already killed more than 2,100 people in the country, making it the second deadliest outbreak of the virus, after the West Africa pandemic of 2014-2016.
Ebola fighters have been hindered by chronic insecurity in the affected provinces of eastern DRC, but much of the controversy surrounding the response has centred on the use of vaccines.
More than 223,000 people living in active Ebola transmission zones have received a vaccination produced by the pharma giant Merck.
WHO has for month been pushing the Kinshasa government to approve the use of a second experimental product, made by Johnson & Johnson, to protect those living outside of direct transmission zones.
The J&J vaccine had been rejected by DRC’s former health minister Oly Ilunga, who cited the risks of introducing a new product in communities where mistrust of Ebola responders is already high.
But Ilunga’s resignation in July appeared to pave the way for approval of the second vaccine.
WHO said in a statement that DRC planned to introduce the J&J product from “mid-October.”
“This vaccine, which is given as a 2-dose course, 56 days apart, will be provided under approved protocols to targeted at-risk populations in areas that do not have active Ebola transmission as an additional tool to extend protection against the virus.”
WHO Director-General, Tedros Adhanom Ghebreyesus, praised the latest decision by DRC authorities, who he said “have once again shown leadership and their determination to end this outbreak as soon as possible”.
‘Rationing’?
Doctors Without Borders, which has repeatedly criticised WHO’s leadership of the Ebola response, levelled fresh criticism against the agency on Monday.
“One of the main problems currently is the fact that, in practice, the (Merck) vaccine is rationed by the WHO and that too few people at risk are protected today,” the charity known by its French acronym MSF said in a statement.
In an interview with AFP in July, MSF’s international president Joanne Liu called on WHO to vaccinate whole villages where Ebola cases had emerged, rather than simply targeting the contacts of those infected.
The charity also renewed its complaints over “the opaque management of the vaccine supplies by the World Health Organization.”
“MSF’s efforts to expand access to vaccination … have been frustrated by the tight controls on supply and eligibility criteria imposed by WHO,” it said in a statement.
“It’s like giving firefighters a bucket of water to put out a fire, but only allowing them to use one cup of water a day,” Natalie Roberts, MSF’s emergency coordinator,” said in a statement.
The charity also called for the creation of “an independent international coordination committee” to “guarantee the transparency of the management of stocks and data sharing”.
‘Everything possible’
WHO denied limiting the availability of the vaccine, saying it was doing “everything possible” to end the epidemic.
“Along with the DRC government, no one wants to bring this epidemic to an end more than WHO,” the agency’s emergency director, Mike Ryan, said in a statement.
WHO is “not limiting access to vaccine but rather implementing a strategy recommended by an independent advisory body of experts,” Ryan added.
The WHO said last week that as of September 17, DRC had registered a total of 3,145 cases of Ebola since the outbreak began over a year ago, including 2,103 deaths.
It has declared the Ebola epidemic a “public health emergency of international concern”, a rare designation used only for the gravest epidemics.






