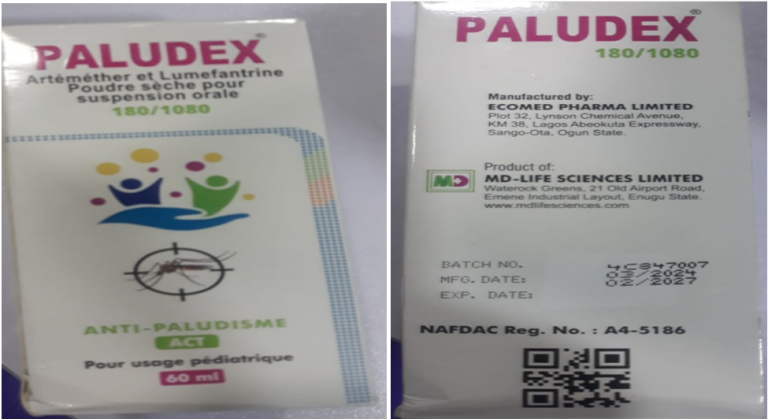
The National Agency for Food, Drugs, Administration and Control (NAFDAC) has notified Nigerians about the sale and distribution of counterfeit Paludex (Artemether/Lumefantrine) (80mg/480mg) and Suspension (20mg/120mg) tablets in circulation.
NAFDAC informed Nigerians about the circulation of these counterfeit tablets via a post shared on its official X handle on Wednesday.
The counterfeit tablets were manufactured by Impact Pharmaceutical Ltd, No. 33A/33B Standard Industrial Layout Emene- Enugu State, Nigeria and marketed by MD Life Sciences Ltd Emene Industrial Layout Enugu State, Nigeria.
READ ALSO: NAFDAC rejects financial inducement allegations in Lung Detox Tea controversy
According to NAFDAC, an analysis run by a WHO-prequalified lab in Germany indicated zero per cent (0%) of Active Pharmaceutical Ingredients (API) in the products.
The same results were obtained when NAFDAC sampled the tablets.
“The results of laboratory analysis by a WHO-prequalified lab in Germany indicated zero per cent (0%) Active Pharmaceutical Ingredients (API) in the products.
“NAFDAC sampled the same products, and the reports of analysis confirmed zero per cent (0%) of API contents.”
It also found Paludex (Artemether/Lumefantrine) dry powder for oral suspension (180mg/1080mg) for paediatric use labelled manufactured by Impact Pharmaceutical Ltd, No. 33A/33B Standard
Industrial Layout Emene- Enugu State, Nigeria and Ecomed Pharma Ltd, Sango-Ota, Ogun State.
These medicines listed by NAFDAC are marketed by MD Life Sciences Ltd Emene Industrial Layout Enugu State, Nigeria.
The agency further noted that the products do not exist on the NAFDAC registered product database, and all the NAFDAC registration numbers stated on the products are false.






