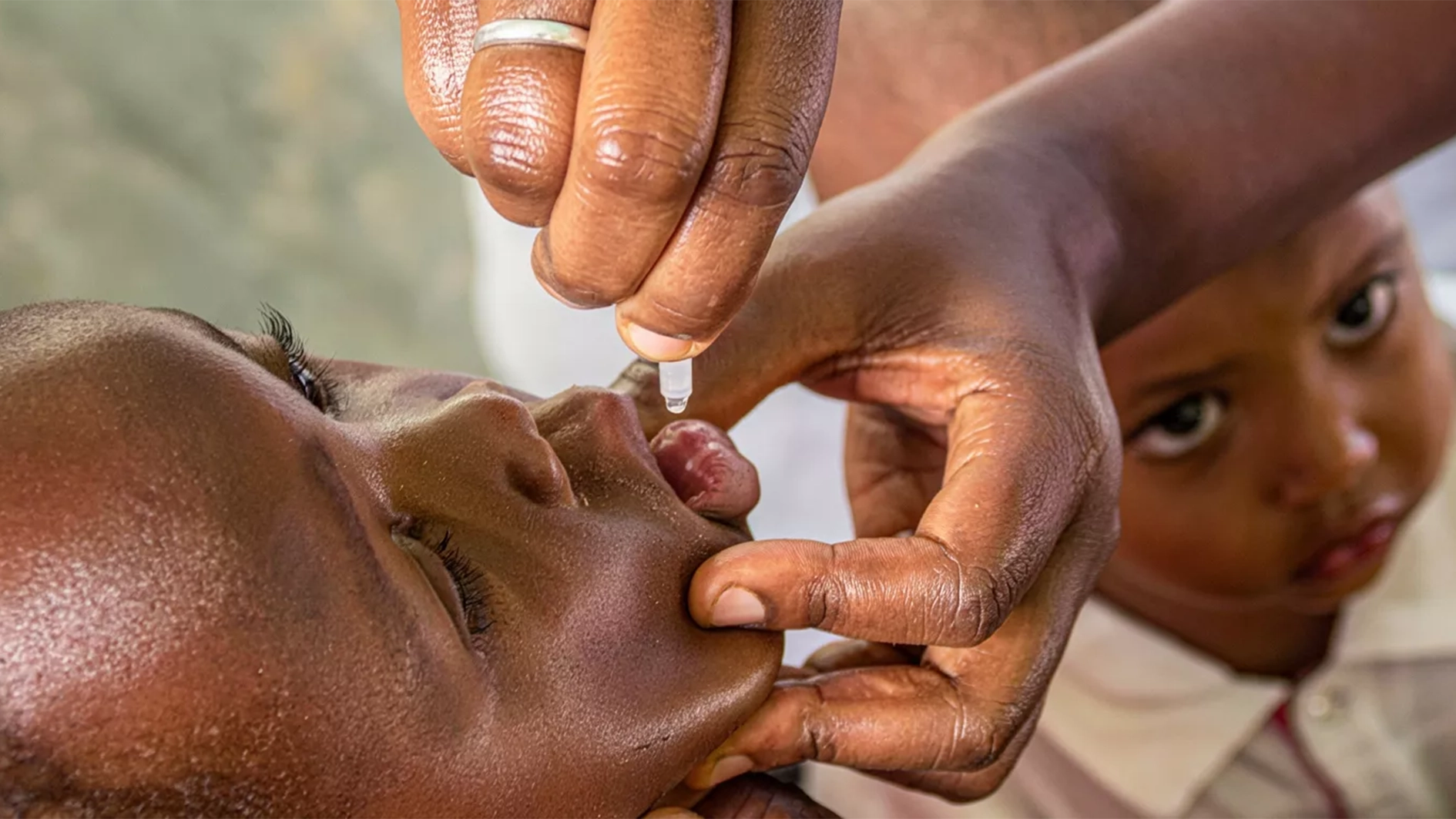
As part of efforts to reduce the country’s high child mortality rate, the Nigerian Institute of Medical Research (NIMR) has begun a nationwide study to determine the safety, effectiveness, and cost-efficiency of administering Azithromycin to children under the age of five.
The study, known as Safety and Antimicrobial Resistance of Mass Administration of Azithromycin Among Children (SARMAAN), was initiated in line with the World Health Organization’s 2022 guidelines.
These guidelines recommended that countries with infant mortality rates above 60 per 1,000 live births and under-five mortality rates exceeding 80 per 1,000 should consider mass administration of Azithromycin (AZM) to infants aged 1–11 months to improve survival rates.
Director of Research at NIMR and a consultant obstetrician-gynecologist, Prof. Oliver Ezechi, explained that Nigeria opted not to implement the World Health Organisation (WHO) recommendation without generating local evidence, prompting the need to develop a comprehensive research framework.
According to Ezechi, the SARMAAN project was designed to ensure the safety of Azithromycin in children, the development of antimicrobial resistance, and financially sustainable intervention. Ezechi noted that while Nigeria aspires to implement robust health interventions, the reality of limited financial resources necessitates careful consideration of cost-effectiveness.
He also pointed out that the project was deliberately designed to avoid creating standalone health programmes that might collapse once external funding ceases. SARMAAN is being implemented through existing delivery platforms such as the National Immunization Programme, the Seasonal Malaria Chemoprevention initiative, and programmes targeting Neglected Tropical Diseases.
The first phase of the study, SARMAAN 1, spanned two years and involved six states selected for cultural and political balance: Akwa Ibom, Abia, Kebbi, Kano, Sokoto, and Jigawa where after baseline surveys were conducted, eligible children received Azithromycin every six months during the study period.
Findings from SARMAAN 1 suggested a favourable safety profile for Azithromycin and no concerning trends in antimicrobial resistance. However, the study was not statistically powered to detect changes in mortality or morbidity rates.
This limitation led to the launch of SARMAAN 2, which has expanded the scope to children aged 1–59 months across 11 northern states with the highest child mortality: Kano, Kaduna, Katsina, Sokoto, Kebbi, Jigawa, Borno, Bauchi, Zamfara, and two others.
Speaking on the challenges, a research fellow and public health expert on the project, Dr Folahanmi Akinsolu, noted that initial delays stemmed from a lack of awareness among policymakers about the role of research in health development, which necessitated multiple rounds of advocacy to the stakeholders.
He also highlighted the problem of limited laboratory infrastructure in the northern regions, which meant that biological samples had to be transported to Lagos for analysis, significantly increasing operational costs. Security concerns further hampered operations, with some areas inaccessible due to conflict or local resistance, and reports of attacks on fieldworkers.
Despite these obstacles, Akinsolu reported steady progress. He noted that the project has continued to gather crucial data, particularly on antimicrobial resistance, in pilot states such as Kano, Kaduna, Bauchi, and Katsina.
Consultant paediatrician and NIMR research fellow Dr Abideen Salako described the SARMAAN initiative as central to NIMR’s mandate of advancing national health through research excellence. He emphasised the strong collaborative framework established by the project, which involves federal and state governments, international academic institutions like the University of Washington and Duke University, and non-governmental partners such as Sight Savers and Solina.
Salako disclosed that SARMAAN 1 successfully delivered 1.8 million doses of Azithromycin across 52 local councils in the participating states. Encouraged by the positive results, states such as Kaduna and Kebbi have committed to full-scale implementation.
He added that beyond its scientific and health goals, the project has created job opportunities and strengthened local engagement, with state health officials playing active roles in its rollout.
According to Salako, community members, particularly mothers have reported noticeable reductions in common childhood illnesses such as diarrhoea, respiratory infections, and skin problems.