The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert over the circulation of falsified Postinor-2 (Levonorgestrel 0.75mg) emergency contraceptive pills in Nigeria, urging consumers and healthcare providers to exercise caution.
In a statement released on August 22, 2025, NAFDAC confirmed that investigations by the Society for Family Health (SFH), the product’s Marketing Authorization Holder (MAH), revealed batches of Postinor-2 in circulation that were not imported by the company and are therefore counterfeit.
The agency identified two types of counterfeit products. Type 1 carries batch number T36184B with a manufacturing date of August 2024 and expiration date of August 2028. Type 2 carries batch number 332, manufactured in March 2023 and expiring in February 2027.


NAFDAC noted visual discrepancies in the packaging, including smaller font sizes on pin verification stickers and misspellings such as “Veify” instead of “Verify” and “Distnibuted in Nigeria” instead of “Distributed in Nigeria.”
“Due to the potential presence of incorrect, substandard, or harmful ingredients, improper dosages of levonorgestrel, and a lack of sterile manufacturing conditions, these falsified products pose significant risks to individual health and public safety,” the statement read.
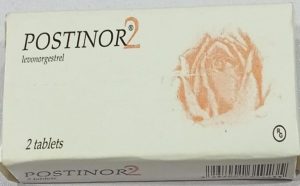
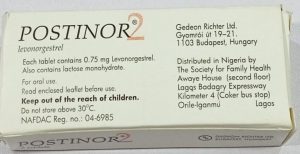
“Risks include failure of contraceptive effect, toxic or harmful contaminants, unpredictable side effects, and potential long-term reproductive health impacts. Counterfeit medicines are unregulated, untested, and illegal, making their safety and efficacy impossible to guarantee.”
NAFDAC has directed all zonal directors and state coordinators to conduct surveillance and remove the counterfeit Postinor-2 products from circulation.
Distributors, retailers, healthcare professionals, and consumers are advised to procure the medicine only from licensed suppliers and to verify the authenticity of the product before use.
Healthcare professionals and the public are encouraged to report any suspected sale of substandard or falsified medicines to the nearest NAFDAC office, via the toll-free line 0800-162-3322, or through email at [email protected].
Adverse reactions related to the product can also be reported through NAFDAC’s e-reporting platforms or the Med-Safety mobile application available for Android and iOS devices.
NAFDAC confirmed that the alert will be shared with the World Health Organization’s Global Surveillance and Monitoring System (GSMS) to support international efforts against counterfeit medicines.
“Patients should only obtain Postinor-2 from verified pharmacies or licensed healthcare providers to ensure safety and efficacy,” the agency advised.