After many decades of ravaging West African countries, especially Nigeria, Lassa fever may soon be contained in the sub-region as scientists from the Coalition for Epidemic Preparedness Initiative (CEPI) and Oxford University, United Kingdom (UK) have developed a vaccine for the disease, which is currently undergoing in-human clinical trials, CHUKWUMA MUANYA writes.
Scientists from the Coalition for Epidemic Preparedness Initiative (CEPI) and Oxford University, United Kingdom (UK) have disclosed how the Ebola Virus disease of 2016 led to the development of the first Lassa fever vaccine that is currently undergoing in-human clinical trials.
The scientists said this during a virtual media chat featuring representatives from CEPI and the University of Oxford to discuss the recently launched Phase One clinical trial of the Oxford Lassa fever vaccine and broader Lassa vaccine preparedness efforts.
They discussed scientific work on Lassa Fever and CEPI’s vaccine development plans; broader Lassa engagement efforts and regional activities; as well as trial details, first volunteer, and Oxford’s vaccine platform.
The experts include, Lassa Disease Programme Lead, CEPI, Dr. Katrin Ramsauer; Head of Lassa Engagement, CEPI, Oyeronke Oyebanji; and Maheshi Ramasamy, an infectious diseases physician and a Professor of Infection and Vaccinology at the University of Oxford (UK).
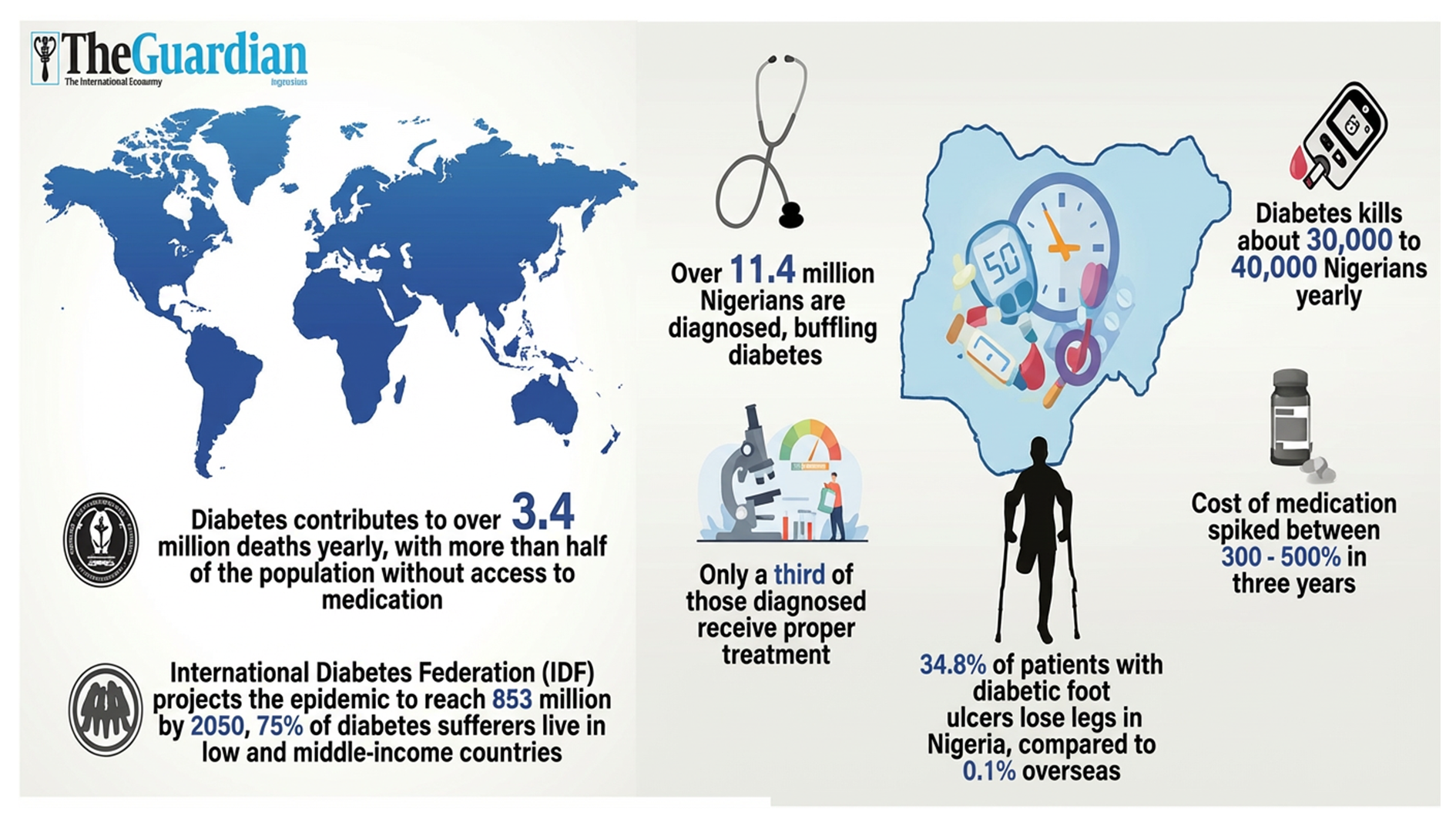
Ramsauer said: “Lassa fever has been around. It was first discovered in 1969. It has been around for over 50 years. It is a long time, and this, you know, is quite a significant public health threat to West Africa, particularly to Nigeria. With sequelae – people losing their hearing – they have long-term neurological sequelae if they survive at all. Very likely, the disease burden is much, much bigger than we had thought.
“So, it was not neglected for anybody, but I think what has happened in the last 10 years was a major outbreak of Ebola in West Africa in 2016. Following this outbreak, it was recognised that there was no funding mechanism globally to develop vaccines against diseases that are a major public health burden, but that have no commercial benefit.”
The first volunteer has received a dose in a first-in-human trial of Oxford’s Lassa vaccine, marking a major milestone in the fight against the deadly virus.
The trial, conducted by the Oxford Vaccine Group and funded by the CEPI, will assess the safety and immune response of the ChAdOx1 Lassa vaccine. Thirty-one people aged 18-55 will participate in the trial in total.
Lassa fever is caused by the Lassa virus, which is primarily spread by rodents and can result in serious illness including deafness, severe bleeding and even death. First discovered in the late 1960s in Nigeria, Lassa fever is endemic in West Africa. The WHO has identified Lassa fever and related viruses as priority pathogens in urgent need of research and development because they pose a significant public health risk due to their potential to cause large outbreaks.
Experts estimate that up to 700 million people could live in regions at risk of Lassa fever by 2070, although there are currently no licenced vaccines or treatments for Lassa fever.
Developed by researchers at the Pandemic Sciences Institute, University of Oxford, the vaccine is made using the same viral vector platform as the Oxford/AstraZeneca COVID-19 vaccine, which is estimated to have saved six million lives in its first year.
Meanwhile, Ramsauer said this was really the birth moment for CEPI. “So, global organisations like Gates Foundation, and countries, put in money to CEPI and said, fund vaccine development for global diseases that can cause pandemics or epidemics.
“And that can help, with a key focus on equitable access. This really is important; so we are not funding vaccines, but generally, we fund vaccines that should be available for those in need.
“And lastly, as a key priority for us, because this is one of the diseases that is only endemic in West Africa; it is a true burden,” she said.
She said CEPI has funded a number of vaccine candidates so far in various stages of development. She also disclosed that one of the vaccine candidates developed by Hayavi, a United States (U.S.) organisation, is in Phase Two clinical trials currently in Nigeria.
Responding to a question on why Ghana instead of Nigeria was chosen for the clinical trials of the latest Lassa fever vaccine, Ramsauer said: “We are working with a number of developers in different countries, and I think it is really critical to understand the safety of the vaccines in the beginning of the development. So, clinical trials in very small scales are done in environments, in partnerships, to be able to allow a rapid development, and then as soon as possible. Oxford will go to Ghana very soon. Ghana has also reported fewer cases.”
She, however, said there are a lot of plans for clinical trials in Nigeria, adding that they have invested in strengthening clinical trial capacity in West African countries, particularly in Nigeria, to make the country ready to kick in with clinical trials once the candidates are available.
On her part, Oyebanji said CEPI, for the last two years, has been working closely with the West African Health Organisation (WAHO) to both design and implement the Lassa Fever Coalition, which is a unique structure that brings together West African governments. She said Nigeria, Liberia, Sierra Leone, Guinea, and Benin Republic are some of the first countries that have signed up to lead this together with WAHO and CEPI, to think about how to accelerate the development of vaccines, and how to make sure that there is an enabling environment for the work that researchers and scientists are doing, like the University of Oxford and several others.
Oyebanji said this work has been going on for the last two years, noting that it is a fixed structure embedded within ministries of health or national public health agencies across the different countries. She said together with, or within the context of the Lassa Fever Coalition, they made some real progress in the last years.
Oyebanji stated that for the first time, they have published the Lassa Fever Policy Research Agenda, which was done through the Coalition, working across a very broad range of colleagues at global, regional, and country level.
“We are currently working with our developers, Oxford and other developers, on a Lassa fever vaccine demand forecast. Again, that is work done through the coalition, so that whatever we’re doing is really embedded in country input. That piece of work is also going to support us with thinking about how a vaccine could be accepted in future; its availability, its pricing, and related questions. And so, I think in summary, CEPI has a very, very strong relationship with West African governments through the Laser Fever Coalition and through other mechanisms. That means that on a day-to-day basis, we are working hand-in-hand; we are thinking about decisions together; we are thinking about the science. But we are also thinking about the future introduction together,” she said.
Reacting to challenges related to trials in terms of vaccine hesitancy and confidence, Oyebanji said: “The first message that I think is important to emphasise is that we continue to learn from previous experiences as we think about Lassa fever because this will not be the first vaccine to be introduced in the country. And if you think about Nigeria, you have a context where, with our routine vaccines, there’s very high acceptance – 80-90 per cent coverage.
“With polio, at some point, we saw strong hesitancy with low coverage. And so, one of the first moves that we are making through a CEPI-funded study is to understand the social science context and community engagement context for Lassa fever vaccines specifically.
“So, through this study that we are funding in Nigeria, in Liberia, and in Sierra Leone, we will look to understand the likelihood of people joining clinical trials. What is the likelihood of acceptance of a vaccine in future? What is community reaction to Lassa fever vaccine? We are starting early because we think it’s important to understand; but we will also continue to do this over time while the vaccine is being introduced, given that the broader context may change. That study is being done as part of our existing Lassa fever epidemiological study. So, the scientists, the communities, the people we work with are already in place, and we are using that to learn more about the social science aspect.”
On the need for more candidate vaccines for Lassa fever, Ramasamy said: “First of all, why do we need another candidate? I mean, as we have seen already with COVID-19, in order to deal with an outbreak, we really need to have resilience in the vaccines that are available to treat an outbreak, which means we need to have more than one vaccine that is available so that we can use it in different populations, that we have the resilience in terms of manufacturing to get enough doses into enough farms.
“So, that’s really the rationale for having more than one candidate vaccine for Lassa fever, and that’s why we are working on a separate vaccine here in Oxford.
“The vaccine is based on the Chadox platform. So, this is a viral vectored platform. So, it takes a chimpanzee cold virus and it incorporates a bit of genetic code of the Lassa fever virus into it. And so this is not in itself infectious, but it is able to stimulate an immune response. And this is the same type of vaccine that was used successfully by the Oxford AstraZeneca COVID-19 vaccine.
“So, we have a lot of information, a lot of understanding and knowledge about this platform. Over two billion doses of that vaccine were used worldwide.
“So, this vaccine has been tested already in what we call preclinical models. So in animal models, and as you have heard, we have now opened the first in-human clinical trial here in Oxford.
“Now, the reason we have started that in Oxford is because we have worked on this platform extensively in Oxford; we have a lot of experience in it. And we are testing it here in our population in Oxford, partly because we have all the downstream laboratory assays set up here for us to be able to understand how well this vaccine stimulates the immune system. So, once we take blood samples from our vaccine participants, we take it to our lab and we can analyse how well that vaccine has stimulated the immune system.”
Ramsamy said they hope to start the next phase of study early next year. “So, the next clinical trial is in Ghana, and we’re working with collaborators in central Ghana to work on that. And they have actually already come to us in Oxford, and they have learned how to develop those assays themselves, and they will be running all the tests themselves in Ghana, in-country, on their participant samples,” she said.
Ramsamy further explained: “Once we’ve run through these first two clinical trials, we then hope to, a bit like the IRV vaccine, expand to later phase trials in Nigeria as well as in other countries. So, that is on our plan. We have already engaged with the Nigeria Lassa Task Force, and we have visits planned to Nigeria next year, as well as other countries to help see how we could set up the next phase of clinical trials in other countries in West Africa. So that’s certainly what we are looking to do.”
Ramsamy said the Oxford study has now immunised six participants, who are all healthy volunteers.
“The way we recruit is that we tell people in the wider Oxford community – students, members of the public – about this study. We advertise this study, and we recruit participants between the ages of 18 and 50 to come and enroll to take part.
“We screen them carefully; we make sure that they are healthy. We do some blood tests; we examine things like their hearing to make sure they are all very healthy and only then are they able to take part in the study.
“So, we have vaccinated six participants already; they are all doing well, and we have our first safety meeting with our safety board next week.
“You will understand that I can’t, as a doctor, tell you any more details about any of our participants in terms of their exact ages or their genders. But they are all Oxford residents. And they are between the ages of 18 and 50, and we have not seen any safety concerns at all with any of them.
“So, this is really encouraging, but it’s a small number of people. It’s only six participants, which is why we are going to enroll another 25 participants here in the UK. In Ghana, we are going to be recruiting around 50 participants to the Phase One study there, and then, as I said, we would then be looking at the next stage of clinical trial vaccine development, Phase Two trials, which we would be hoping to do in Nigeria as well as other countries.”