Aina said this in an interview with the News Agency of Nigeria (NAN) on Monday in Abuja.
He said that while Nigeria continued to monitor and manage the spread of Mpox, vaccination remained one of the most effective methods of protection, especially for individuals at higher risk of exposure.
“The Mpox vaccine is not just for those who have been exposed; it plays a vital role in pre-exposure prophylaxis.
READ ALSO:North Central APC Elders Forum hails Sule over conflict resolution in zone
“This means that getting vaccinated can significantly reduce the risk of infection before any potential exposure to the virus,” he said.
He said that the NPHCDA had been working in collaboration with the Federal Ministry of Health and other partners to ensure that vaccines were available and accessible.
He urged the public, particularly those in high-risk categories, to take advantage of the vaccination campaigns being conducted across the country.
“We are committed to ensuring that all Nigerians, particularly healthcare workers, individuals in affected communities, and those with compromised immune systems, are protected against Mpox..
“Vaccination is a critical part of our strategy to curb the spread of the virus,” he said.
According to the NPHCDA boss, the agency has also been engaging in public health awareness campaigns to educate citizens about the benefits of the Mpox vaccine and the importance of early vaccination.
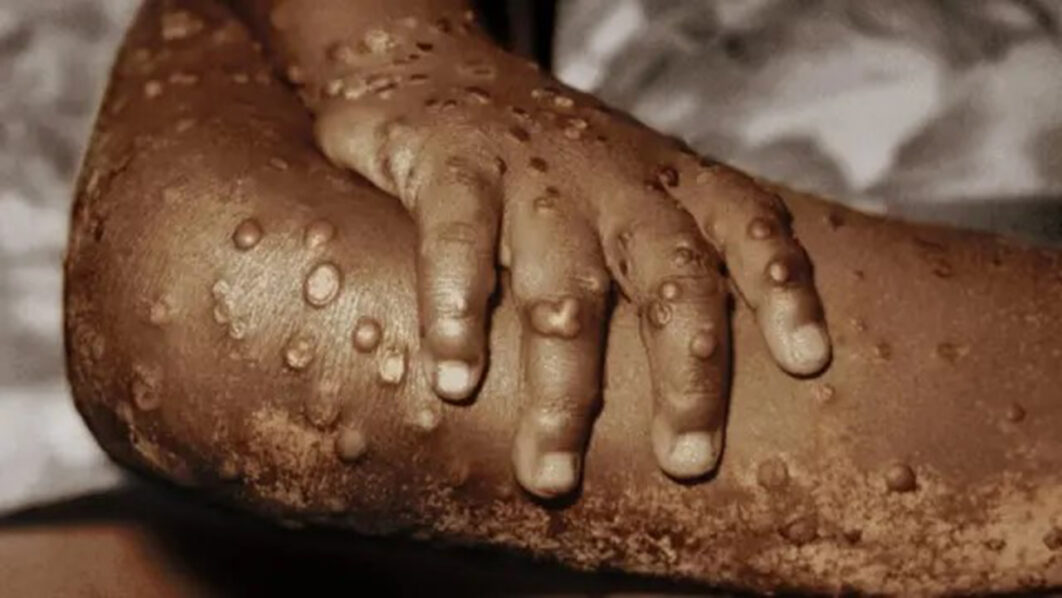
He said that Mpox was a viral disease that could cause a range of symptoms, including fever, rash, and swollen lymph nodes.
“The virus can spread through close contact with an infected person or animal, or through contaminated materials.
“Although Mpox is generally self-limiting, it can lead to severe complications, particularly in immunocompromised individuals.
“As Nigeria continues its efforts to combat the spread of Mpox, the NPHCDA’s vaccination initiatives remain a cornerstone of the country’s public health response,” he said.
He said that the country would begin vaccinations for mpox from October 8, after regulatory approvals are concluded.
He said that the country received the first batch of 10,000 doses of the Mpox vaccine from the U.S. Agency for International Development on Tuesday.
READ ALSO:FG prosecutes illegal mining suspects in Kogi, Ondo
“The country has confirmed 48 Mpox cases with no fatalities yet.
“Due to limited available doses 9,980 of the Jynneos MPox vaccine, the quantities will be split evenly 1,996 doses across the five states for implementation,”he said.
He said the vaccines would be subjected to regulatory laboratory analysis for three weeks after which they would be distributed across five states with 4,750 persons receiving two doses each, 28 days apart.
The NPHCDA boss said that the target population for the vaccinations would be those in close contact with Mpox cases, health workers and persons with low immune status.
NAN, reports that JYNNEOS® is a third-generation vaccine licensed to prevent smallpox and Mpox
It is recommended by the Advisory Committee on Immunization Practices (ACIP) for individuals at risk of exposure to orthopox virus infections, including Mpox.
READ ALSO:Probe petrol importation scam now, Falana tells FG
Since the 2022 Mpox outbreak, JYNNEOS has been the primary vaccine used in the U.S. It is based on the live, attenuated Modified Vaccinia Ankara (MVA) virus, which does not replicate efficiently in humans.
JYNNEOS is also known as Imvamune® or Imvanex® internationally and is fully licensed in the U.S for use in adults aged 18 and older.
It became commercially available in the U.S as of April 1, 2024.