*Centre woos NIMR on treating advanced solid tumours including metastatic cancers
*Scientists cure blindness with pioneering treatment that repairs damaged retina
*Researchers report second example of transplant in clinic leading to HIV remission
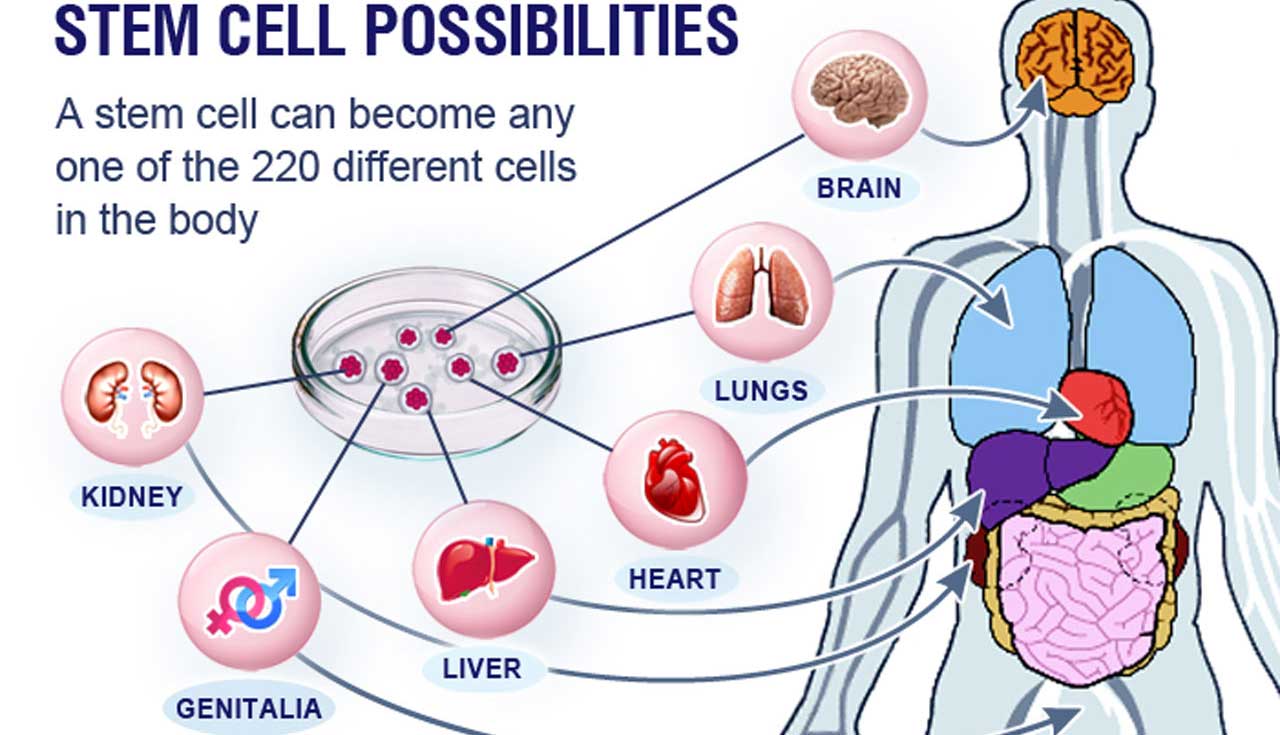
There is hope in sight of a cure for Nigerians living with cancer, vision impairment, arthritis, type 1 and 2 diabetes, sexual dysfunction, and neurological disorders like acute non-haemorrhagic stroke, and spinal cord injuries.
The Glory Wellness & Regenerative Centre with clinics in United States (U.S.), Lagos and Abuja is wooing the Nigerian Institute for Medical Research (NIMR) Yaba, Lagos for clinical trails using stem cell therapy to treat chronic diseases.
Until now, physicians all over the world are increasingly employing stem cell therapies for the treatment of chronic diseases including Human Immuno-deficiency Virus (HIV), hypertension, diabetes, chronic kidney disease, neurological disorders, asthma, diabetes, rheumatoid arthritis, spinal cord injuries, female and male sexual dysfunction, joint pain and autoimmune disease.
One of the pioneers of stem cell therapy in Nigeria and the Medical Director of Glory Wellness & Regenerative Centre, Dr. David Ikudayisi, told The Guardian: “I started preliminary talk late last year with NIMR in Yaba about doing research on treating advanced solid tumours including metastatic cancers using the combination of the currently proven safe adult stem cell therapy and a currently proven safe drug/medication. In USA, combining two independently safe procedures or drugs for new indications require clinical trials. You cannot just assume that the combination is safe because they are safe individually without doing clinical trials.”
Ikudayisi said the study or clinical trial is already going on in United Kingdom (U.K.), Germany and USA, Nigeria and Poland are fighting to be number four and five clinical trial centres in the world using this new protocol that involves safe autologous adult stem cell therapy and a save drug, though the drug is not readily available to the physicians.
Ikudayisi is a United States (U.S.) Board Certified Internist with a strong passion for regenerative aesthetic and cosmetic medicine. He said if all goes well, by early next year, may be sooner, Nigeria may be among the few centres in the world for this safe clinical trials as no side effect reported yet and the trial is already in state two going to stage three in those three centres mentioned earlier. “So I am exited for Nigeria if and when we actual jump over the current hurdle holding us back. Nigeria can become a medical tourist centre in Africa for treating or curing advanced solid tumors with or without metastasis with a protocol that has no side effect. You cannot beat that,” Ikudayisi said.
Have you used stem cell therapy to cure spinal cord injury? Ikudayisi explained: “I have a video showing the progress of a patient who suffered a complete spinal cord injury. He arrived at the Glory Wellness & Regenerative Centre not being able to move his upper and lower extremities, except for some painful contractions in the legs and slight movement in his right hand. He received a stem cell therapy treatment: adult adipose-derived stem cell therapy. This safe procedure has shown promise in the past to help the body regenerate and enable previously lost functions.
“He started showing improvements in less than two days. His contractions reduced drastically from five-six times hourly to about two times the entire day!
This video shows his progress after the treatment through the course of over one year. He was able to gain more control of his upper extremities. Regenerative medicine is helping everyday people to regain functions that were once thought to be permanently lost.”
Ikudayisi said that there are several thousand clinical trials based on autologous (patient’s own) Mesenchymal Stem Cells (MSCs). He said these type of stem cells are relatively easy to obtain from a patient via bone marrow blood or fat tissue and have been shown to hold vast healing potential.
Ikudayisi said Adult Stem Cell Therapy (ASCT) and Platelet Rich Plasma Therapy (PRPT) are under a new specialty of medicine known as regenerative medicine, which is a specialist segment of medicine that helps people to naturally regenerate and rejuvenate their bodies from the different conditions they may be suffering from without using chemicals or the orthodox medicine we are used to.
Also, victims of a devastating form of blindness have been given hope by a new stem-cell treatment that rejuvenates the eyes.
There is no cure for retinitis pigmentosa, an inherited condition that slowly constricts vision, but a British firm has reported early success with a revolutionary procedure that helps to repair a damaged retina.
The treatment involves growing billions of ‘progenitor’ stem cells in a laboratory. These have the ability to transform themselves into other types of cell depending on where they are placed in the body.
A million stem cells are injected into the back of the patient’s eyeball. Once there, they transform themselves into new light-sensitive cells called rods and cones, which replace those lost prematurely to genetic flaws.
Tests on three patients – two men and a woman – who were legally blind produced ‘exciting’ results, according to Olav Hellebo, chief executive of United Kingdom (U.K.) biotech firm ReNeuron.
Before the procedure, the three could read only the largest group of letters on a special eye test chart, but 18 days after being injected with the cells, their sight had improved to the point where they could read three letter sizes smaller.
One patient achieved sufficient progress to no longer be classified as legally blind and another told her doctor that she was able to see the food on her plate for the first time in years.
Also, in a first-of-its-kind trial in the United States, researchers are testing a stem cell-derived natural killer cell immunotherapy in people with incurable cancer.
With the advent of immunotherapy, researchers hoped to boost a person’s immune system to fight and destroy tumors effectively. Although this type of therapy has completely changed the treatment landscape for cancers such as melanoma, there remain a significant number of people whose tumors can evade their immune system.
Joining the likes of adoptive cell transfer and checkpoint inhibitors on the list of immunotherapies are natural killer (NK) cells. These specialized white blood cells come equipped with a potent armory of tools to make short work of cancer cells.
Now, researchers at the University of California (UC) San Diego School of Medicine, United States, are running a clinical trial with industrial collaborator Fate Therapeutics to investigate NK cells both alone and in combination with checkpoint inhibitors in people with advanced solid tumours.
One particular factor sets this study apart from others using NK cells for similar purposes.
This “off-the-shelf” NK immunotherapy trial is the first in the U.S. to use cells that the researchers have derived from induced pluripotent stem (iPS) cells.
Back in 2013, Dr. Dan Kaufman — professor of medicine in the Division of Regenerative Medicine and director of cell therapy at UC San Diego School of Medicine — and his team developed a method of expanding large numbers of NK cells from human iPS cells for cancer therapy.
They published the method in the journal Stem Cells Translational Medicine.
After extensive preclinical testing, the US Food and Drug Administration (FDA) gave Dr. Kaufman and Fate Therapeutics the go-ahead last November to set up a phase I clinical trial to test their iPS-derived NK immunotherapy in people with advanced solid tumours.
The phase I trial started in February and will include up to 64 people with advanced, untreatable cancer. The main aim of the trial is to assess the safety of the treatment. The other objectives are to determine the extent to which the tumors respond to NK cell therapy and to find out how long the cells stay in the participants’ bodies.
It will be a while before the results of the trial are available, with expectations being that the study will run into 2022. However, it paves the way not only for a new generation of immunotherapies to treat cancer but also for other iPS-derived cell therapies to follow.
Ikudayisi further explained: “ASCT may hold answers to many questions and problems that we doctors believed had no solutions, especially neurological disorders. Adult stem cell therapy with or without PRPT revitalizes and regenerates the body organs and systems; it also reverses and repairs many pending subclinical medical problems before they become apparent, including the diseases that are age-related.”
He said that ASCT and PRPT are safe as shown by many published research reports and clinical trials done already. He, however, said this does not guarantee that adverse effects cannot occur if physicians that are not properly trained do the treatment.
The US-trained said ASCT has helped a lot of people all over the world to regain their lives back from debilitating ailments and Nigerians are not left behind. He said there are real people in Nigeria that were either wheelchair bound but now walking freely with occasional use of a cane or using a cane before but now walking without one; diabetes patients are able to have restoration of vision in their eyes, and some feel and look younger.
He said ASCT has helped chronic kidney disease patients in Nigeria that are on haemodialysis to either reduce the frequency of haemodialysis per week or like in a patient that was recommended to have kidney transplant a year ago is now off haemodialysis and off diabetic medications, and remain stable for the last six months.
Ikudayisi said men with erectile dysfunction are now feeling like young men again. He further explained: “I would be remiss to mention that the type of treatment protocol, the dosage of stem cells used also play a role in the efficacy of the treatment, and not everyone will respond in the same manner. Most of the patients showed improvements after the first treatment, and the few that needed second treatment went on to see great results after more treatments were done; needless to say that they were elated with the results.
“The only groups of patients that will always need more than a couple of transplantation sessions are patients with the neurological disorders. The latest researches and evidence-based studies show the number of treatment session needed to get significant clinical results can decreased by adding Exosomes to the treatment sessions.”
Ikudayisi said there are diseases that conventional treatments have no cure for, but ASCT can reverse the symptoms of those diseases, repair, and regenerate the damaged tissues or organs involved.
Also, a second example reported of a stem-cell transplant in the clinic leading to HIV remission.
A person infected with HIV who was treated for blood cancer with a stem-cell transplant has gone into viral remission, with no trace of the virus in their blood. A similar outcome in 2009 had not been replicated until now.
HIV infects immune cells, and the current standard treatment is long-term use of antiretroviral drugs. This keeps virus levels low in the bloodstream but doesn’t eradicate HIV from cells in the body. In 2009, it was reported that a person with HIV (commonly referred to as the Berlin patient) who was treated for cancer using a stem-cell transplant subsequently went into viral remission — the virus became undetectable in their body, even in the absence of antiretroviral therapy. No other cases of long-term HIV remission occurring in this way had been recorded since then. But now, writing in Nature, Gupta et al. report a person who has achieved HIV remission for at least 18 months.
The case reported by Gupta and colleagues is similar in many ways to the one described a decade ago. Both individuals had developed immune-cell cancer and received stem cell transplants from donors (who were not infected with HIV) to re-establish their immune-cell populations. Both donors had a mutation (termed Δ32) in both of their copies of a gene called CCR5. This gene encodes a receptor protein on immune cells that HIV can bind to during the process of infection. Having a Δ32 mutation in both cellular copies of the CCR5 gene results in the absence of functional CCR5 protein on the cell surface, and immune cells lacking this protein can resist infection by HIV strains that depend on CCR5. Both patients were infected with HIV strains that exclusively use CCR5, along with the protein CD4, to aid cellular entry, which was probably a key factor in explaining why stem-cell treatment resulted in HIV remission.
Gupta et al. report the case of an HIV-infected person who underwent cancer treatment and then went into long-term HIV remission — the virus was undetectable in their bloodstream even in the absence of antiretroviral drug treatment — for at least 18 months. This confirms an outcome reported in 2009, which had not been repeated until now.
Also, a revolutionary treatment may prevent blindness. Scientists have discovered a way of boosting stem cells’ healing properties after eye injuries and acid attacks.
Acid attack victims could be saved from blindness after a ‘revolutionary’ treatment, which has been tested by scientists. The pioneering method uses an enzyme, which prevents stem cells – blank cells, which can turn into new tissue – from losing their healing abilities in the eye following injury. Stem cells treat an injury to the eye, such as from acid burns, cuts or infection, by dividing and heading directly to the wound.
The development offers hope to almost 500,000 people across the world each year that lose their sight due to chemical burns. One of the most famous cases of this in the United Kingdom (UK) was that of TV personality Katie Piper who was attacked in 2008 and needed some of her sight restored.
Using live corneal tissues from an eye in a lab, researchers from Newcastle University and the University of Missouri, United States (U.S.), recreated the effects of chemical burns. They found stem cells could lose their healing properties when the tissue they’re contained in ‘stiffens’ after an injury. They found that using collagenase, a tissue-softening enzyme, could soften up the stiffened area. This, in turn, made it more able to support stem cells to promote healing.
The study authors described the research as being ‘a potential revolution’ in its field. The research was published in the journal Nature Communications.
Dr. Ricardo Gouveia said: “This study demonstrates a potential new way to treat injuries by changing the stiffness of the natural environment which we have shown changes the behaviour of the adult stem cells.”
When stem cells repair a damaged cornea they quickly divide in great numbers and become cells, which can make a solid membrane then move to the injury to seal it. However, this healing process can be compromised when injuries reach the stem cell niche – the area of tissue in the cornea where stem cells live.
The scientists found that stiffening the niche causes stem cells to mature and lose their self-renewing properties.
Professor Che Connon, from Newcastle University, said: “We can now prove that the cornea becomes stiffer when exposed to injuries such as those caused by what are commonly known as acid attacks.
“[We can] demonstrate that wound healing is impaired due to stem cells differentiating in response to the stiffening of their otherwise soft niche, and not because they are killed during injury, as previously thought. “This is an exciting development in the field of corneal biology, and allows us to better understand how vision works. “But even more important, it provides us with a new set of strategies to treat eye conditions which were until now inoperable.”
Both the US Food and Drug Administration and the European Medicine Agency have already approved the collagenase formulation for other purposes. The scientists said the findings are also promising for people suffering from blindness caused by corneal scarring from cuts or disease.
The cornea is a thin piece of tissue, which can be easily damaged by injury or infection, sometimes by contact lenses or a fingernail. More than 1.5 million new cases of corneal blindness are reported every year worldwide, and around 4,000 cornea transplants are done every year in the UK. Professor Connon said their study is even relevant to the research of cancer treatment, where tumour stiffening is a known marker of aggressive cancer cell behaviour.






